by Jose Antonio PhD. PREFACE – It has been about a score (that’s 20 years for those who slept through English 101) since I’ve plastered my myopic eyeballs on to a dissecting microscope and counted skeletal muscle fibers. Counting muscle fibers is as painful as watching the afternoon gabfest known as The View (yeah, you understand). Actually scratch that. The View is much more painful. Either way, I wanted to shed light on a topic that is of interest to many; yet very few are aware of the research that has been published. I’m one of a handful (perhaps a half-dozen or less) of science nerds that has actually done research on muscle fiber hyperplasia. To do that, you got to count. And believe me my friends, you are counting all day and frickin’ night. It’s like watching ants march in a line. If you stare at them long enough, you’ll go cross-eyed and eventually blind. Okay, maybe not blind. Seriously, why do we do this kind of research? Ultimately, the question that is always asked is as follows: Do human beings have the capacity to increase the number of skeletal muscle fibers? And if so, what’s the mechanism? For answers to that, read on my friend.
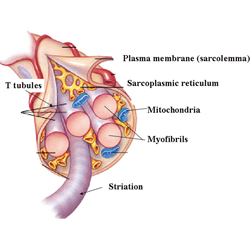
WHAT IS HYPERPLASIA? Hypertrophy refers to an increase in the size of the cell while hyperplasia refers to an increase in the number of cells or fibers. A single muscle cell is usually called a fiber, muscle fiber or myofiber. Ok. You passed Cell Biology 101. Whew.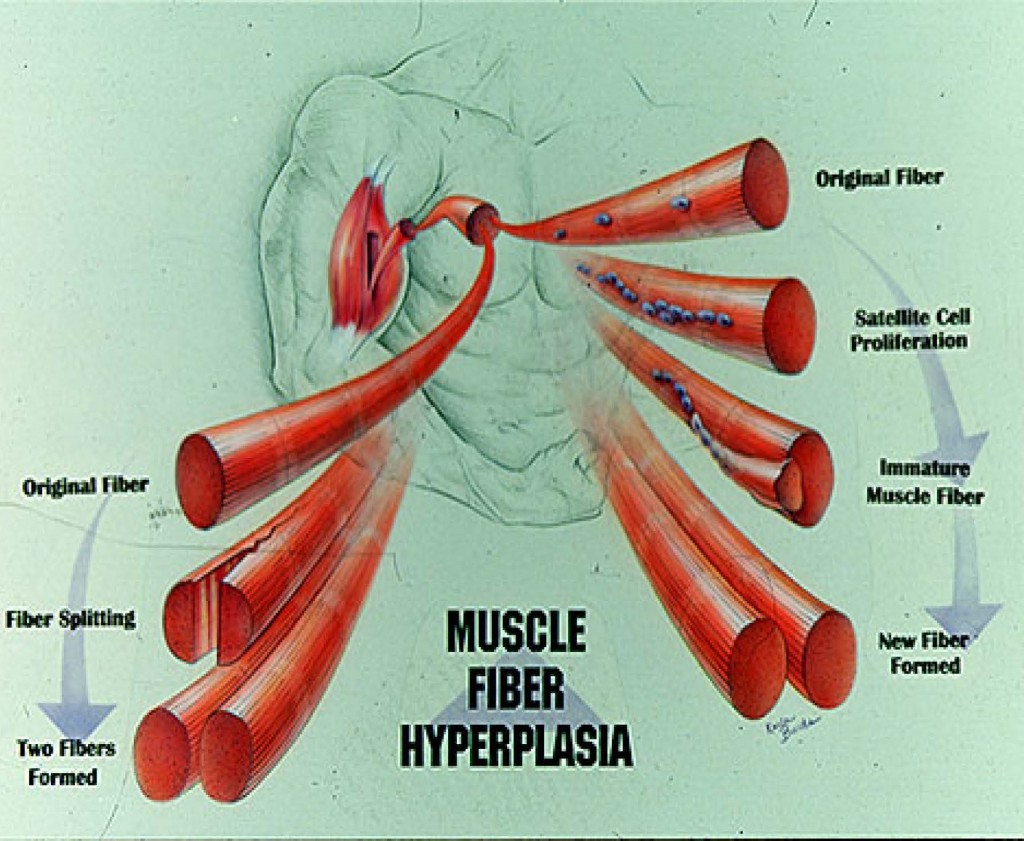
HOW DO MUSCLE FIBERS ADAPT TO DIFFERENT TYPES OF EXERCISE? If you look at a good marathon runner’s physique and compared him/her to a bodybuilder it becomes obvious that training specificity has a profound effect. We know that aerobic training results in an increase in mitochondrial volume/density, oxidative enzymes, and capillary density [1]. Also, in some elite endurance athletes the trained muscle fibers may actually be smaller than those of a completely untrained person. Bodybuilders and other strength-power athletes, on the other hand, have much larger muscles [2, 3]. That’s their primary adaptation; their muscles get bigger. All the cellular machinery related to aerobic metabolism (i.e., mitochondria, oxidative enzymes, etc.) isn’t necessary for maximal gains in skeletal muscle force output; in essence, you just need more contractile protein. We know that this muscle mass increase is due primarily to fiber hypertrophy; however, are there situations where muscles also respond by increasing fiber number? Ok, you now know the basics of training specificity. For now, go to the head of the class.
EVIDENCE FOR SKELETAL MUSCLE FIBER HYPERPLASIA – Scientists have come up with all  sorts of methods to study muscle growth in laboratory animals. You might wonder what relevance this has to humans. Keep in mind that some of the procedures which scientists perform on animals simply can’t be done on humans due to ethical and logistical reasons. Unless of course you support human sacrifice like the ancient Aztecs. Nonetheless, the more convincing data supporting muscle fiber hyperplasia emerges from animal studies. Some human studies have also suggested the occurence of muscle fiber hyperplasia. I’ll address those studies later. Unless you fall asleep first.
sorts of methods to study muscle growth in laboratory animals. You might wonder what relevance this has to humans. Keep in mind that some of the procedures which scientists perform on animals simply can’t be done on humans due to ethical and logistical reasons. Unless of course you support human sacrifice like the ancient Aztecs. Nonetheless, the more convincing data supporting muscle fiber hyperplasia emerges from animal studies. Some human studies have also suggested the occurence of muscle fiber hyperplasia. I’ll address those studies later. Unless you fall asleep first.
DOES “STRETCH” INDUCE FIBER HYPERPLASIA? Please don’t confuse ‘stretch’ with doing yoga in your Lululemon pants. Not THAT kind of stretching. Instead, it’s a more painful type we’re talking about here. The avian stretch model was first used by Sola et al. in 1973 [4]. In essence, you put a weight on one wing of a bird (usually a chicken or quail) and leave the other wing alone. By putting a weight on one wing (usually equal to 10% of the bird’s weight), a weight-induced stretch is imposed on the back muscles. The muscle which is usually  examined is the anterior latissimus dorsi or ALD (unlike humans, birds have an anterior and posterior latissimus dorsi). Besides the expected observation that the individual fibers grew under this stress, Sola et al. found that this method of overload resulted in a 16% increase in ALD muscle fiber number. Since the work of Sola, numerous smarty-pants science types have used this model [5-20]. For example, Alway et al. [5] showed that 30 days of chronic stretch (i.e., 30 days with the weight on with NO REST) resulted in a 172% increase in ALD muscle mass and a 52-75% increase in muscle fiber number! Imagine if humans could grow that fast. Yeah. And imagine if Kate Upton shows up at your next birthday party.
examined is the anterior latissimus dorsi or ALD (unlike humans, birds have an anterior and posterior latissimus dorsi). Besides the expected observation that the individual fibers grew under this stress, Sola et al. found that this method of overload resulted in a 16% increase in ALD muscle fiber number. Since the work of Sola, numerous smarty-pants science types have used this model [5-20]. For example, Alway et al. [5] showed that 30 days of chronic stretch (i.e., 30 days with the weight on with NO REST) resulted in a 172% increase in ALD muscle mass and a 52-75% increase in muscle fiber number! Imagine if humans could grow that fast. Yeah. And imagine if Kate Upton shows up at your next birthday party.
I also performed a study using the avian stretch model. However, I put a significant twist on this model [10]. I used a progressive overload scheme whereby the ALD was initially loaded with a weight equal to 10% of the bird’s body weight followed by increments of 15%, 20%, 25%, and 35% (of the bird’s body weight) (5). Each weight increment was interspersed with a 2-day rest. The total number of stretch days was 28. Using this approach produced the greatest gains in muscle mass EVER recorded in an animal or human model of tension-induced overload, up to a 334% increase in muscle mass with up to a 90% increase in fiber number. I was also able to uncouple the hypertrophic and hyperplastic responses. Meaning that the muscle fibers undergoing progressive stretch overload actually increased muscle fiber size at first, and then underwent hyperplasia secondarily. Perhaps the muscle fibers reached a critical cell size (upon which further increases in muscle fiber cross-sectional areas would have compromised the ability of the cell to obtain nutrients). If you look at the light micrograph below, you will see that ginormous fiber in panel “B” labeled with an asterisk. Just think. Back then, the word ginormous didn’t exist. Now I actually have an excuse to use the word. Anyhow, that muscle fiber is the largest EVER in the published literature. But you’ll notice the fissures in it (see the arrow pointing at the fissure). Perhaps it’s splitting into smaller parts. That’s evidence of muscle fiber splitting. I mean come on. You can only get SO BIG.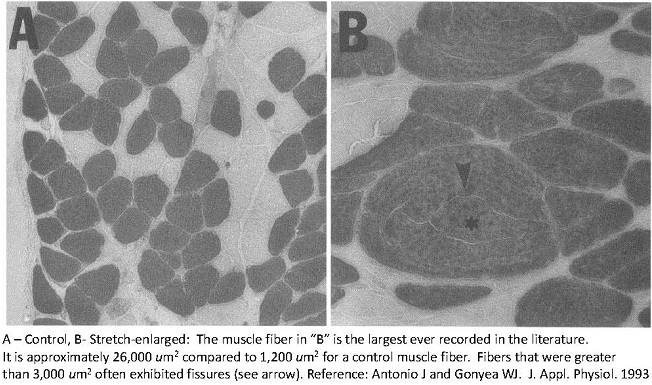
But you might ask yourself, what does hanging a weight on a bird have to do with humans who lift weights? So who cares if birds can increase muscle mass by over 300% and fiber number by 90%. To the naked eye, you seem to have a good point. But if you care about the mechanisms that regulate skeletal muscle size, than I would highly recommend you drink a bit more coffee and pay attention. Certainly, nobody out there hangs weights on their arms for 30 days straight or even 30 minutes for that matter. Maybe you should try it and see what happens. This could be a different albeit painful way to “train.” But actually the physiologically interesting point is that if presented with an appropriate stimulus, a muscle can produce more fibers. What is an appropriate stimulus? I think it is one that involves subjecting muscle fibers to high tension overload (enough to induce injury) followed by a regenerative period. Can you get hypertrophy without injury or damage? Yes. I’d surmise that inducing actual damage to the sarcolemma, Z-lines, etc is the ‘best’ way to ultimately promote growth.
SIDE BAR – Intraset Stretching by Jacob Wilson PhD – Dr Wilson (at the University of Tampa) has taken the basic science work that I’ve done and applied it to the human condition. Check this out! http://www.bodybuilding.com/fun/jake-wilsons-project-mass-intraset-stretching.html
WHAT ABOUT EXERCISE? The stretch induced method is a rather unusual stimulus compared to normal muscle activity. What about “normal” muscular exercise? Several scientists have used various models of ‘tension overload’ to study the role of muscle fiber hyperplasia in muscular growth [5-11, 18-39]. Dr. William Gonyea was the first to demonstrate exercised-induced muscle fiber hyperplasia using weight-lifting cats as the model [25, 28, 29, 40]. Cats were trained to perform a wrist flexion exercise with one forelimb against resistance in order to receive a food reward. The non-trained forelimb thus served as a control for comparison. Resistance was increased as the training period progressed. He found that in addition to hypertrophy, the forearm muscle (flexor carpi radialis) of these cats increased fiber number from 9-20%. After examining the training variables that predicted muscle hypertrophy the best, scientists from Dr. Gonyea’s laboratory found that lifting speed had the highest correlation to changes in muscle mass (i.e., cats which lifted the weight in a slow and deliberate manner made greater muscle mass gains than cats that lifted ballistically) [41].
Keep in mind that these cats trained in a normal manner. It wasn’t like they were doing crazy high volume or weight. Thus, it would seem reasonable that heavy resistance training (when done reasonably hard) in humans could also result in gains in muscle fiber number. To suggest that you need to kill yourself by training like a maniac just isn’t supported by the cat weight-lifting data.
Rats have also been used to study muscle growth [32, 34, 42]. In a model developed by Japanese researchers [34], rats performed a squat exercise in response to an electrical stimulation. They found that fiber number in the plantaris muscle (a plantar flexor muscle on the posterior side of the leg) increased by 14%. Moreover, an interesting observation has been made in hypertrophied muscle which suggests the occurrence of muscle fiber 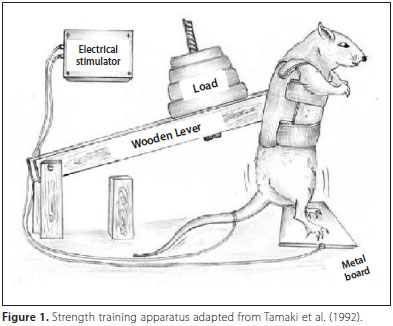 hyperplasia [16, 22, 23, 42, 43]. Individual small fibers have been seen frequently in enlarged muscle. Initially, some researchers believed this to be a sign of muscle fiber atrophy. However, it doesn’t make any sense for muscle fibers to atrophy while the muscle as a whole hypertrophies. Instead, it seems more sensible to attribute this phenomenon to de novo formation of muscle fibers (i.e., these are newly made fibers). I believe this is another piece of evidence, albeit indirect, which supports the occurrence of muscle fiber hyperplasia.
hyperplasia [16, 22, 23, 42, 43]. Individual small fibers have been seen frequently in enlarged muscle. Initially, some researchers believed this to be a sign of muscle fiber atrophy. However, it doesn’t make any sense for muscle fibers to atrophy while the muscle as a whole hypertrophies. Instead, it seems more sensible to attribute this phenomenon to de novo formation of muscle fibers (i.e., these are newly made fibers). I believe this is another piece of evidence, albeit indirect, which supports the occurrence of muscle fiber hyperplasia.
EXERCISE-INDUCED GROWTH IN HUMANS – The main problem with human studies to determine if muscle fiber hyperplasia contributes to muscle hypertrophy is the inability to make direct counts of human muscle fibers. Some would rather stick a fork in their eye than count muscle fibers. And a mere perusal through the published literature shows indeed how rare studies are that do direct muscle fiber counts or count the fibers in an entire histological cross-section(s). Heck, you’d have a better chance of finding a McDonalds in North Korea than finding a graduate student or PhD willing to do muscle fiber counts. For instance, one study  determined that the tibialis anterior muscle contains approximately 160,000 fibers. Imagine counting 160,000 fibers [44] for just one muscle! The biceps brachii muscle contains roughly a quarter of a million muscle fibers [45]. One study found it to be as high as 418,884 [46].
determined that the tibialis anterior muscle contains approximately 160,000 fibers. Imagine counting 160,000 fibers [44] for just one muscle! The biceps brachii muscle contains roughly a quarter of a million muscle fibers [45]. One study found it to be as high as 418,884 [46].
SIDE BAR – Do we lose muscle fibers with age? Apparently we don’t. Amen to that! According to a study by Klein et al: “We have compared the number of muscle fibers in the biceps brachii muscle (BB) of six old men (82.3 +/- 4.3 years) and six young men (21.2 +/- 1.9 years). Muscle fiber number was estimated by dividing the maximal area of the BB, determined with magnetic resonance imaging, by the mean fiber area of the BB determined in a muscle biopsy. The percentage of type II fibers in the BB (approximately 60%) and the type I fiber area were not different between the groups. The BB area (-26%), type II fiber area (-24%), mean fiber area (-20%), and maximal voluntary contraction strength (MVC) of the elbow flexor muscles (-27%) were lower in the old than young group. However, the estimated number of muscle fibers was not significantly different between the young (253000) and old (234000) men. Consequently, the smaller BB area of the old men could be explained primarily by a smaller type II fiber size. These findings suggest that old age is not associated with a reduced number of muscle fibers in the BB. The relative contribution of a reduction in fiber number to age-related muscle atrophy may be muscle-dependent[45].”
So how do human studies come up with evidence for hyperplasia? It’s arrived at in an indirect fashion. For instance, one study showed that elite bodybuilders and powerlifters had arm circumferences 27% greater than normal sedentary controls yet the size (i.e., cross-sectional area) of athlete’s muscle fibers (in the triceps brachii m.) were not different than the control group. The investigators stated that “Despite large differences in elbow extension strength and arm girth there was no significant difference in fibre areas or percentages of fibre types between the elite group and the trained controls[47].” This of course suggests that the elite group had a greater number of skeletal muscle fibers. Nygaard and Neilsen [48] did a cross-sectional study in which they found that swimmers had smaller Type I and IIa fibers in the deltoid muscle when compared to controls despite the fact that the overall size of the deltoid muscle was greater. Larsson and Tesch [49] found that bodybuilders possessed thigh circumference measurements 19% greater than controls yet the average size of their muscle fibers were not different from the controls. Furthermore, Alway et al. [26] compared the biceps brachii muscle in elite male and female bodybuilders. They showed that the cross-sectional area of the biceps muscle was correlated to both fiber area and number. Other studies, on the other hand, have demonstrated that bodybuilders have larger fibers instead of a greater number of fibers when compared to a control population [46, 50, 51]. Some scientists have suggested that the reason many bodybuilders or other athletes have muscle fibers which are the same size (or smaller) versus untrained controls is due to a greater genetic endowment of muscle fibers. That is, they were born with more fibers. If that was true, then the intense training over years and decades performed by elite bodybuilders has produced at best average size fibers. That means, some bodybuilders were born with a bunch of below average size fibers and training enlarged them to average size. I don’t know about you, but I’d find that explanation rather tenuous. Actually, that explanation is just plain dopey. It would seem more plausible (and scientifically defensible) that the larger muscle mass seen in bodybuilders is due primarily to muscle fiber hypertrophy but also to fiber hyperplasia. So the question that needs to be asked is not whether muscle fiber hyperplasia occurs, but rather under what conditions does it occur. I believe the the scientific evidence shows clearly in animals, and indirectly in humans, that fiber number can increase. Does it occur in every situation where a muscle is enlarging? No. But can it contribute to muscle mass increases? Yes.
Nygaard and Neilsen [48] did a cross-sectional study in which they found that swimmers had smaller Type I and IIa fibers in the deltoid muscle when compared to controls despite the fact that the overall size of the deltoid muscle was greater. Larsson and Tesch [49] found that bodybuilders possessed thigh circumference measurements 19% greater than controls yet the average size of their muscle fibers were not different from the controls. Furthermore, Alway et al. [26] compared the biceps brachii muscle in elite male and female bodybuilders. They showed that the cross-sectional area of the biceps muscle was correlated to both fiber area and number. Other studies, on the other hand, have demonstrated that bodybuilders have larger fibers instead of a greater number of fibers when compared to a control population [46, 50, 51]. Some scientists have suggested that the reason many bodybuilders or other athletes have muscle fibers which are the same size (or smaller) versus untrained controls is due to a greater genetic endowment of muscle fibers. That is, they were born with more fibers. If that was true, then the intense training over years and decades performed by elite bodybuilders has produced at best average size fibers. That means, some bodybuilders were born with a bunch of below average size fibers and training enlarged them to average size. I don’t know about you, but I’d find that explanation rather tenuous. Actually, that explanation is just plain dopey. It would seem more plausible (and scientifically defensible) that the larger muscle mass seen in bodybuilders is due primarily to muscle fiber hypertrophy but also to fiber hyperplasia. So the question that needs to be asked is not whether muscle fiber hyperplasia occurs, but rather under what conditions does it occur. I believe the the scientific evidence shows clearly in animals, and indirectly in humans, that fiber number can increase. Does it occur in every situation where a muscle is enlarging? No. But can it contribute to muscle mass increases? Yes.
HOW DOES MUCLE FIBER HYPERPLASIA OCCUR? There are two primary mechanisms in
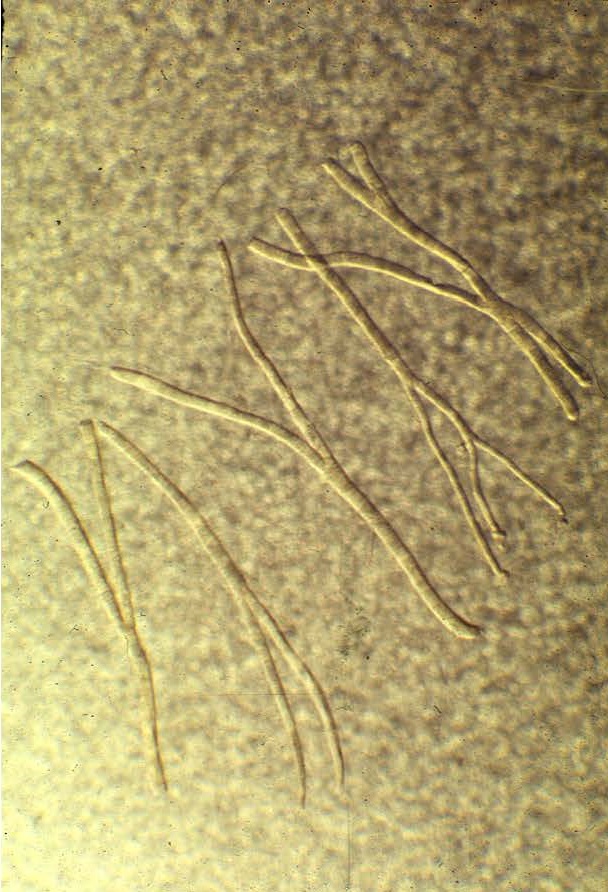
Check out these ‘split’ or branching fibers.
which new fibers can be formed. First, large fibers can split into two or more smaller fibers (i.e., fiber splitting) [8, 10, 32, 34]. And perhaps the primary mechanism is via the activation and proliferation of satellite cells [18-20, 23, 52, 53]. Satellite cells are myogenic stem cells which are involved in skeletal muscle regeneration. When you injure, stretch, or severely exercise a muscle fiber, satellite cells are activated [18-20, 53]. Satellite cells proliferate (i.e., undergo mitosis or cell division) and give rise to new myoblastic cells (i.e., immature muscle cells). These new myoblastic cells can either fuse with an existing muscle fiber causing that fiber to get bigger (i.e., hypertrophy) or these myoblastic cells can fuse with each other to form a new fiber (i.e., hyperplasia).
ROLE OF MUSCLE FIBER DAMAGE – There is robust evidence which has shown the importance of eccentric contractions in producing muscle hypertrophy [54-57]. It is known that eccentric contractions produces greater injury than concentric or isometric contractions. We also know that if you can induce muscle fiber injury, satellite cells are activated. Both animal and human studies point to the superiority of eccentric contractions in increasing muscle mass [55-57]. However, in the real world, we don’t do pure eccentric, concentric, or isometric contractions. We do a combination of all three. So the main thing to keep in mind when performing an exercise is to allow a controlled descent of the weight 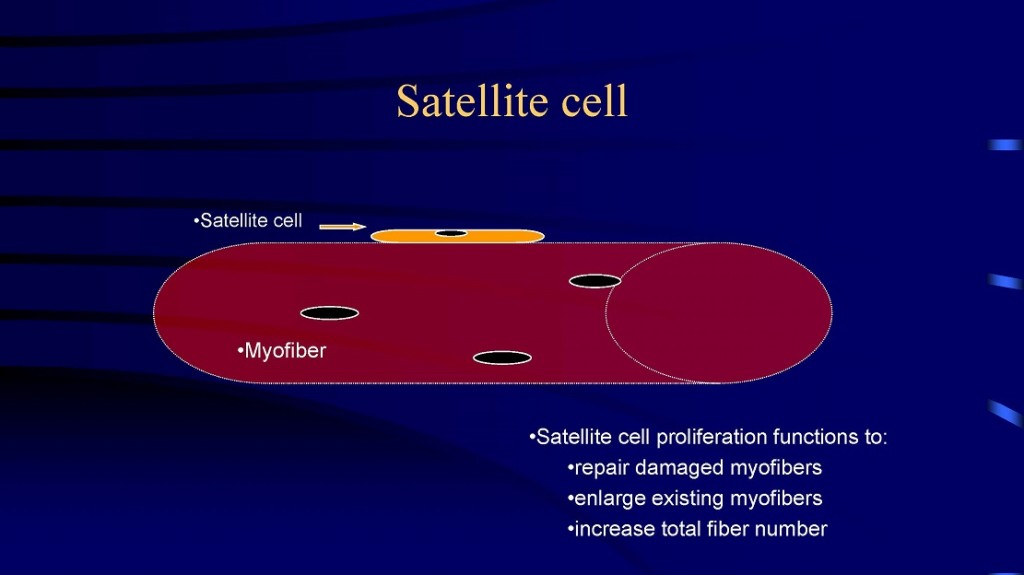 being lifted. And on occasion, one could have his/her training partner load more weight than can be lifted concentrically and spot him/her while he/she performs a pure eccentric contraction. This will really put your muscle fibers under a great deal of tension causing microtears and severe delayed-onset muscle soreness. Thus, the repeated process of injuring your fibers (via weight training) followed by a recuperation or regeneration may result in an overcompensation of protein synthesis resulting in a net anabolic effect [58][59].
being lifted. And on occasion, one could have his/her training partner load more weight than can be lifted concentrically and spot him/her while he/she performs a pure eccentric contraction. This will really put your muscle fibers under a great deal of tension causing microtears and severe delayed-onset muscle soreness. Thus, the repeated process of injuring your fibers (via weight training) followed by a recuperation or regeneration may result in an overcompensation of protein synthesis resulting in a net anabolic effect [58][59].
SIDE BAR: THE MYTH OF SARCOPLASMIC VS MYOFIBRILLAR HYPERTROPHY – This is perhaps one of the more annoying and contrived controversies in the field of skeletal muscle plasticity. Was it Joseph Goebbels who said that “if you repeat a lie often enough, it becomes the truth”? This is a perfect example of this maxim. Frankly, I don’t give a shit who started this mythical beast known as sarcoplasmic hypertrophy. The fact of the matter is there has never been evidence to suggest that increases in skeletal muscle size can be the result of selective hypertrophy of the sarcoplasm. It’s not like you can train to increase the sarcoplasm one day; and then the next day, train for myofibrillar growth. Roughly 70-80% of the volume of a muscle fiber is the myofibrillar component. It should be as clear as the Montana sky that gains in muscle size are largely due to increases in contractile protein. In fact there is a classic paper by Claassen et al. (Journal of Physiology, 1989, 409: 491-495) entitled “Muscle Filament Spacing and Short-term Heavy-Resistance Exercise in Humans.” They found that the “linear distance between myofilaments as well as the ratio of actin to myosin filament did not change with training.”  So the next time you hear a bodybuilder or training guru talk about achieving greater ‘muscle density,’ they’re just making shit up. And while you’re at it, if you like your doctor you can keep your doctor. And if you like your healthcare plan, you can keep it. Moreover, don’t confuse gains in strength without gains in skeletal muscle size as evidence for sarcoplasmic hypertrophy. As my teenage daughter would say, ‘isn’t it obvy?’ Yes, it is obvy (obvious) that improvements in rate coding, muscle activation patterns and motor unit recruitment do indeed account for changes in strength without a concomitant increase in skeletal muscle size. However, if someone shows me evidence that an enlarged muscle or muscle fiber has an increase in cytoplasm with no change in the volume of actin and myosin, then I’ll alter my conclusions. Until then, show me the evidence. And if it doesn’t exist, then quit bs’ing everyone about ‘sarcoplasmic’ hypertrophy. What’s next? Pigs flying, unicorns trotting, the Jets winning? For another opinion, check out Stu Phillips PhD little ditty: https://twitpl.us/t/4Xeb
So the next time you hear a bodybuilder or training guru talk about achieving greater ‘muscle density,’ they’re just making shit up. And while you’re at it, if you like your doctor you can keep your doctor. And if you like your healthcare plan, you can keep it. Moreover, don’t confuse gains in strength without gains in skeletal muscle size as evidence for sarcoplasmic hypertrophy. As my teenage daughter would say, ‘isn’t it obvy?’ Yes, it is obvy (obvious) that improvements in rate coding, muscle activation patterns and motor unit recruitment do indeed account for changes in strength without a concomitant increase in skeletal muscle size. However, if someone shows me evidence that an enlarged muscle or muscle fiber has an increase in cytoplasm with no change in the volume of actin and myosin, then I’ll alter my conclusions. Until then, show me the evidence. And if it doesn’t exist, then quit bs’ing everyone about ‘sarcoplasmic’ hypertrophy. What’s next? Pigs flying, unicorns trotting, the Jets winning? For another opinion, check out Stu Phillips PhD little ditty: https://twitpl.us/t/4Xeb
HAS THE HYPERPLASIA DEBATE BEEN SETTLED? To quote the genius of Yogi Berra, “I wish I had an answer to that because I’m tired of answering that question.” In my scientific opinion, this issue has already been settled. Muscle fiber hyperplasia can contribute to whole muscle hypertrophy. There is human as well as rat, cat, and bird data which support this proposition [6-10, 20, 23, 25, 28, 32-34, 47, 60-66], a veritable wild kingdom of evidence. Does muscle fiber hyperplasia occur under all circumstances? No. There are several studies which show no change in fiber number despite significant increases in muscle mass [11, 13, 37, 67]. Is it possible that certain muscles can increase fiber number more so than others? Maybe. Can any Joe Schmoe off the street who lifts weights to get in better shape increase the number of fibers for instance in their biceps? Maybe, maybe not. What about the elite bodybuilder who at 5’8″ tall is ripped at a body weight of 250 lbs.? Are his large muscles purely the result of muscle fiber hypertrophy? I think it would be extremely naive to think that the massive size attained by elite bodybuilders is due solely to fiber hypertrophy. Despite the contention that fiber number is constant once you’re born, there is an abundance of evidence which shows that muscle fiber number can increase post-natally.

Always wondered how much hyperplasia could occur in the gluteus maximus. One, two, 5999, 190000, 699699, oh my…keep counting.
Besides, there is nothing magical at birth which says that now that you’re out of the womb, you can no longer make more muscle fibers. A mechanism exists for muscle fiber hyperplasia and there is plenty of reason to believe that it occurs. Of course, the issue is not whether fiber number increases after every training program, stress, or perturbation is imposed upon an animal (or human). The issue is again, under which circumstances is it most likely to occur. For humans, I’d surmise that the average person who lifts weights and increases their muscle mass moderately probably won’t induce fiber hyperplasia in their exercised muscle(s). Yet I imagine there are exceptions. However, the elite bodybuilder who attains the massive muscular development now seen may be the more likely candidate for exercise-induced muscle fiber hyperplasia. If you are interested in a comprehensive scientific treatise on this subject, read a scientific review article that I wrote way back when Bill Clinton was President of the USA [9]. It still holds true today.
REFERENCES YOU SHOULD READ BUT PROBABLY WON’T. NETFLIX WINS ALL THE TIME.
1. Holloszy JO, Booth FW: Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 1976, 38:273-291.
2. Costill DL, Coyle EF, Fink WF, Lesmes GR, Witzmann FA: Adaptations in skeletal muscle following strength training. J Appl Physiol Respir Environ Exerc Physiol 1979, 46:96-99.
3. Tesch PA, Larsson L: Muscle hypertrophy in bodybuilders. Eur J Appl Physiol Occup Physiol 1982, 49:301-306.
4. Sola OM, Christensen DL, Martin AW: Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol 1973, 41:76-100.
5. Alway SE, Winchester PK, Davis ME, Gonyea WJ: Regionalized adaptations and muscle fiber proliferation in stretch-induced enlargement. J Appl Physiol (1985) 1989, 66:771-781.
6. Alway SE, Gonyea WJ, Davis ME: Muscle fiber formation and fiber hypertrophy during the onset of stretch-overload. Am J Physiol 1990, 259:C92-102.
7. Antonio J, Gonyea WJ: Ring fibres express ventricular myosin in stretch overloaded quail muscle. Acta Physiol Scand 1994, 152:429-430.
8. Antonio J, Gonyea WJ: Muscle fiber splitting in stretch-enlarged avian muscle. Med Sci Sports Exerc 1994, 26:973-977.
9. Antonio J, Gonyea WJ: Skeletal muscle fiber hyperplasia. Med Sci Sports Exerc 1993, 25:1333-1345.
10. Antonio J, Gonyea WJ: Progressive stretch overload of skeletal muscle results in hypertrophy before hyperplasia. J Appl Physiol (1985) 1993, 75:1263-1271.
11. Antonio J, Gonyea WJ: Role of muscle fiber hypertrophy and hyperplasia in intermittently stretched avian muscle. J Appl Physiol (1985) 1993, 74:1893-1898.
12. Ashmore CR, Summers PJ: Stretch-induced growth in chicken wing muscles: myofibrillar proliferation. Am J Physiol 1981, 241:C93-97.
13. Gollnick PD, Parsons D, Riedy M, Moore RL: Fiber number and size in overloaded chicken anterior latissimus dorsi muscle. J Appl Physiol Respir Environ Exerc Physiol 1983, 54:1292-1297.
14. Barnett JG, Holly RG, Ashmore CR: Stretch-induced growth in chicken wing muscles: biochemical and morphological characterization. Am J Physiol 1980, 239:C39-46.
15. Holly RG, Barnett JG, Ashmore CR, Taylor RG, Mole PA: Stretch-induced growth in chicken wing muscles: a new model of stretch hypertrophy. Am J Physiol 1980, 238:C62-71.
16. Kennedy JM, Eisenberg BR, Reid SK, Sweeney LJ, Zak R: Nascent muscle fiber appearance in overloaded chicken slow-tonic muscle. Am J Anat 1988, 181:203-215.
17. McCormick KM, Schultz E: Mechanisms of nascent fiber formation during avian skeletal muscle hypertrophy. Dev Biol 1992, 150:319-334.
18. Winchester PK, Gonyea WJ: Regional injury and the terminal differentiation of satellite cells in stretched avian slow tonic muscle. Dev Biol 1992, 151:459-472.
19. Winchester PK, Gonyea WJ: A quantitative study of satellite cells and myonuclei in stretched avian slow tonic muscle. Anat Rec 1992, 232:369-377.
20. Winchester PK, Davis ME, Alway SE, Gonyea WJ: Satellite cell activation in the stretch-enlarged anterior latissimus dorsi muscle of the adult quail. Am J Physiol 1991, 260:C206-212.
21. Armstrong RB, Marum P, Tullson P, Saubert CWt: Acute hypertrophic response of skeletal muscle to removal of synergists. J Appl Physiol Respir Environ Exerc Physiol 1979, 46:835-842.
22. Chalmers GR, Roy RR, Edgerton VR: Variation and limitations in fiber enzymatic and size responses in hypertrophied muscle. J Appl Physiol (1985) 1992, 73:631-641.
23. Giddings CJ, Gonyea WJ: Morphological observations supporting muscle fiber hyperplasia following weight-lifting exercise in cats. Anat Rec 1992, 233:178-195.
24. Gollnick PD, Timson BF, Moore RL, Riedy M: Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol Respir Environ Exerc Physiol 1981, 50:936-943.
25. Mikesky AE, Giddings CJ, Matthews W, Gonyea WJ: Changes in muscle fiber size and composition in response to heavy-resistance exercise. Med Sci Sports Exerc 1991, 23:1042-1049.
26. Alway SE, Grumbt WH, Gonyea WJ, Stray-Gundersen J: Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol (1985) 1989, 67:24-31.
27. Giddings CJ, Neaves WB, Gonyea WJ: Muscle fiber necrosis and regeneration induced by prolonged weight-lifting exercise in the cat. Anat Rec 1985, 211:133-141.
28. Gonyea WJ: Role of exercise in inducing increases in skeletal muscle fiber number. J Appl Physiol Respir Environ Exerc Physiol 1980, 48:421-426.
29. Gonyea WJ: Muscle fiber splitting in trained and untrained animals. Exerc Sport Sci Rev 1980, 8:19-39.
30. Gonyea WJ, Ericson GC: Morphological and histochemical organization of the flexor carpi radialis muscle in the cat. Am J Anat 1977, 148:329-344.
31. Gonyea WJ, Ericson GC: An experimental model for the study of exercise-induced skeletal muscle hypertrophy. J Appl Physiol 1976, 40:630-633.
32. Ho KW, Roy RR, Tweedle CD, Heusner WW, Van Huss WD, Carrow RE: Skeletal muscle fiber splitting with weight-lifting exercise in rats. Am J Anat 1980, 157:433-440.
33. Gonyea WJ, Sale DG, Gonyea FB, Mikesky A: Exercise induced increases in muscle fiber number. Eur J Appl Physiol Occup Physiol 1986, 55:137-141.
34. Tamaki T, Uchiyama S, Nakano S: A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc 1992, 24:881-886.
35. Eddinger TJ, Moss RL, Cassens RG: Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fisher 344 rats. J Histochem Cytochem 1985, 33:1033-1041.
36. Timson BF, Dudenhoeffer GA: The effect of severe dietary protein restriction on skeletal muscle fiber number, area and composition in weanling rats. J Anim Sci 1985, 61:416-422.
37. Timson BF, Bowlin BK, Dudenhoeffer GA, George JB: Fiber number, area, and composition of mouse soleus muscle following enlargement. J Appl Physiol (1985) 1985, 58:619-624.
38. Dudenhoeffer GA, Bowlin BK, Timson BF: A brief study of within litter and within strain variation in skeletal muscle fiber number in three lines of laboratory rodents. Growth 1985, 49:450-454.
39. Vaughan HS, Goldspink G: Fibre number and fibre size in a surgically overloaded muscle. J Anat 1979, 129:293-303.
40. Gonyea WJ: Fiber size distribution in the flexor carpi radialis muscle of the cat. Anat Rec 1979, 195:447-454.
41. Mikesky, A. E., W. Matthews, C. J. Giddings, and W. J. Gonyea. Muscle enlargement and exercise performance in the cat. J. Appl. Sport Sci. Res. 3: 85-92, 1989.
42. Yamada S, Buffinger N, DiMario J, Strohman RC: Fibroblast growth factor is stored in fiber extracellular matrix and plays a role in regulating muscle hypertrophy. Med Sci Sports Exerc 1989, 21:S173-180.
43. Kennedy JM, Sweeney LJ, Gao LZ: Ventricular myosin expression in developing and regenerating muscle, cultured myotubes, and nascent myofibers of overloaded muscle in the chicken. Med Sci Sports Exerc 1989, 21:S187-197.
44. Frenzel H, Schwartzkopff B, Reinecke P, Kamino K, Losse B: Evidence for muscle fiber hyperplasia in the septum of patients with hypertrophic obstructive cardiomyopathy (HOCM). Quantitative examination of endomyocardial biopsies (EMCB) and myectomy specimens. Z Kardiol 1987, 76 Suppl 3:14-19.
45. Klein CS, Marsh GD, Petrella RJ, Rice CL: Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve 2003, 28:62-68.
46. MacDougall JD, Sale DG, Alway SE, Sutton JR: Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol Respir Environ Exerc Physiol 1984, 57:1399-1403.
47. MacDougall JD, Sale DG, Elder GC, Sutton JR: Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur J Appl Physiol Occup Physiol 1982, 48:117-126.
48. Nygaard, E. and E. Nielsen. Skeletal muscle fiber capillarisation with extreme endurance training in man. In Eriksson B, Furberg B (Eds). Swimming Medicine IV(vol. 6, pp. 282-293). University Park Press, Baltimore, 1978.
49. Larsson L, Tesch PA: Motor unit fibre density in extremely hypertrophied skeletal muscles in man. Electrophysiological signs of muscle fibre hyperplasia. Eur J Appl Physiol Occup Physiol 1986, 55:130-136.
50. Haggmark T, Jansson E, Svane B: Cross-sectional area of the thigh muscle in man measured by computed tomography. Scand J Clin Lab Invest 1978, 38:355-360.
51. Schantz P, Fox ER, Norgren P, Tyden A: The relationship between the mean muscle fibre area and the muscle cross-sectional area of the thigh in subjects with large differences in thigh girth. Acta Physiol Scand 1981, 113:537-539.
52. Bischoff R: Interaction between satellite cells and skeletal muscle fibers. Development 1990, 109:943-952.
53. Darr KC, Schultz E: Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol (1985) 1987, 63:1816-1821.
54. Cote C, Simoneau JA, Lagasse P, Boulay M, Thibault MC, Marcotte M, Bouchard C: Isokinetic strength training protocols: do they induce skeletal muscle fiber hypertrophy? Arch Phys Med Rehabil 1988, 69:281-285.
55. Hather BM, Tesch PA, Buchanan P, Dudley GA: Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 1991, 143:177-185.
56. Wong TS, Booth FW: Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol (1985) 1990, 69:1718-1724.
57. Wong TS, Booth FW: Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol (1985) 1990, 69:1709-1717.
58. Carlson BM: The regeneration of skeletal muscle. A review. Am J Anat 1973, 137:119-149.
59. MacDougall, J.D. Morphological changes in human skeletal muscle following strength training and immobilization. In: Human Muscle Power (pp. 269-288). N.L. Jones, N. McCartney, A. J. McComas (Eds.). Human Kinetics Publisher, Inc. Champaign, Illinois, 1986.
60. Roman WJ, Alway SE: Stretch-induced transformations in myosin expression of quail anterior latissimus dorsi muscle. Med Sci Sports Exerc 1995, 27:1494-1499.
61. Carson JA, Alway SE, Yamaguchi M: Time course of hypertrophic adaptations of the anterior latissimus dorsi muscle to stretch overload in aged Japanese quail. J Gerontol A Biol Sci Med Sci 1995, 50:B391-398.
62. Carson JA, Yamaguchi M, Alway SE: Hypertrophy and proliferation of skeletal muscle fibers from aged quail. J Appl Physiol (1985) 1995, 78:293-299.
63. Alway SE: Stretch induces non-uniform isomyosin expression in the quail anterior latissimus dorsi muscle. Anat Rec 1993, 237:1-7.
64. Alway SE: Perpetuation of muscle fibers after removal of stretch in the Japanese quail. Am J Physiol 1991, 260:C400-408.
65. Sjostrom M, Lexell J, Eriksson A, Taylor CC: Evidence of fibre hyperplasia in human skeletal muscles from healthy young men? A left-right comparison of the fibre number in whole anterior tibialis muscles. Eur J Appl Physiol Occup Physiol 1991, 62:301-304.
66. Tamaki T, Akatsuka A, Tokunaga M, Ishige K, Uchiyama S, Shiraishi T: Morphological and biochemical evidence of muscle hyperplasia following weight-lifting exercise in rats. Am J Physiol 1997, 273:C246-256.
67. McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ: Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol (1985) 1996, 81:2004-2012.
About the Author – Jose Antonio PhD is the CEO of the ISSN, www.theissn.org. His published work is in the area of sports nutrition and skeletal muscle plasticity. When he’s not writing, he’s probably watching football or MMA. Or better yet, he’s probably on the ocean paddling like a ninja master. 
 butter on a hot stove. Ok, I put the blue whale part in there. Blue whales can apparently consume half a million calories in one mouthful. Whoa. Can you say Thanksgiving buffet with every bite?! So does making those bi’s, tri’s and glutes a tad bit larger result in resting metabolism that’s copious, capacious or colossal? Or is this Much Ado About Nothing? Before I provide a teleological explanation of why this notion is cockeyed, here’s some food for thought.1, 2 According to Robert R Wolfe PhD, “every 10-kg difference in lean mass translates into a difference in energy expenditure of ~100 kcal/d.” Or in units us Americans prefer, that’s about an increase of 4.5 calories for every pound gained.2 Regardless of whether that number is entirely accurate, let’s just say RMR per unit of fat free mass is about as impressive as dunking a basketball on an 8 foot hoop.
butter on a hot stove. Ok, I put the blue whale part in there. Blue whales can apparently consume half a million calories in one mouthful. Whoa. Can you say Thanksgiving buffet with every bite?! So does making those bi’s, tri’s and glutes a tad bit larger result in resting metabolism that’s copious, capacious or colossal? Or is this Much Ado About Nothing? Before I provide a teleological explanation of why this notion is cockeyed, here’s some food for thought.1, 2 According to Robert R Wolfe PhD, “every 10-kg difference in lean mass translates into a difference in energy expenditure of ~100 kcal/d.” Or in units us Americans prefer, that’s about an increase of 4.5 calories for every pound gained.2 Regardless of whether that number is entirely accurate, let’s just say RMR per unit of fat free mass is about as impressive as dunking a basketball on an 8 foot hoop.
 water or electricity. Man was left to fend for itself like any other wild animal. It helped having a big brain because there was no chance in hell that humans could use physical strength or speed to kill its next meal. Conversely, humans developed quite the endurance capacity to ‘out-work’ its prey and a brain to ‘out-think’ just about every creature on Earth. You’ll notice that even modern day hunter-gatherer societies are ‘endurance’ oriented. You need endurance just to stay alive. Having large muscles serves no survival benefit.
water or electricity. Man was left to fend for itself like any other wild animal. It helped having a big brain because there was no chance in hell that humans could use physical strength or speed to kill its next meal. Conversely, humans developed quite the endurance capacity to ‘out-work’ its prey and a brain to ‘out-think’ just about every creature on Earth. You’ll notice that even modern day hunter-gatherer societies are ‘endurance’ oriented. You need endurance just to stay alive. Having large muscles serves no survival benefit. increased caloric need of 250-500 calories (according to the urban legend figure). Now where would a caveman get these calories? Would he open the fridge to get a protein shake? Go to Burger King and order a Whopper? Duh. It makes zero evolutionary sense for RMR to go up to any appreciable extent if you gain lean body mass. Or put another way. It is energetically costly to gain and maintain skeletal muscle mass. Why? Because you’d have to feed it you knucklehead. It makes evolutionary sense that it is difficult to gain muscle (which it is) and easy to gain fat (which it is). Why? So you can survive the next go round of the zombie apocalypse when an asteroid the size of Hawaii blasts Earth into a nuclear-winter oblivion. If that happens and you survive, you better pray that you have the genes for putting on fat easily. Otherwise, you and all the Victoria’s Secret models will shrivel to death in a matter of weeks.
increased caloric need of 250-500 calories (according to the urban legend figure). Now where would a caveman get these calories? Would he open the fridge to get a protein shake? Go to Burger King and order a Whopper? Duh. It makes zero evolutionary sense for RMR to go up to any appreciable extent if you gain lean body mass. Or put another way. It is energetically costly to gain and maintain skeletal muscle mass. Why? Because you’d have to feed it you knucklehead. It makes evolutionary sense that it is difficult to gain muscle (which it is) and easy to gain fat (which it is). Why? So you can survive the next go round of the zombie apocalypse when an asteroid the size of Hawaii blasts Earth into a nuclear-winter oblivion. If that happens and you survive, you better pray that you have the genes for putting on fat easily. Otherwise, you and all the Victoria’s Secret models will shrivel to death in a matter of weeks.